Fast shipping by DHL
Search suggestions
No search results.
Items
No search results.
Categories
No search results.
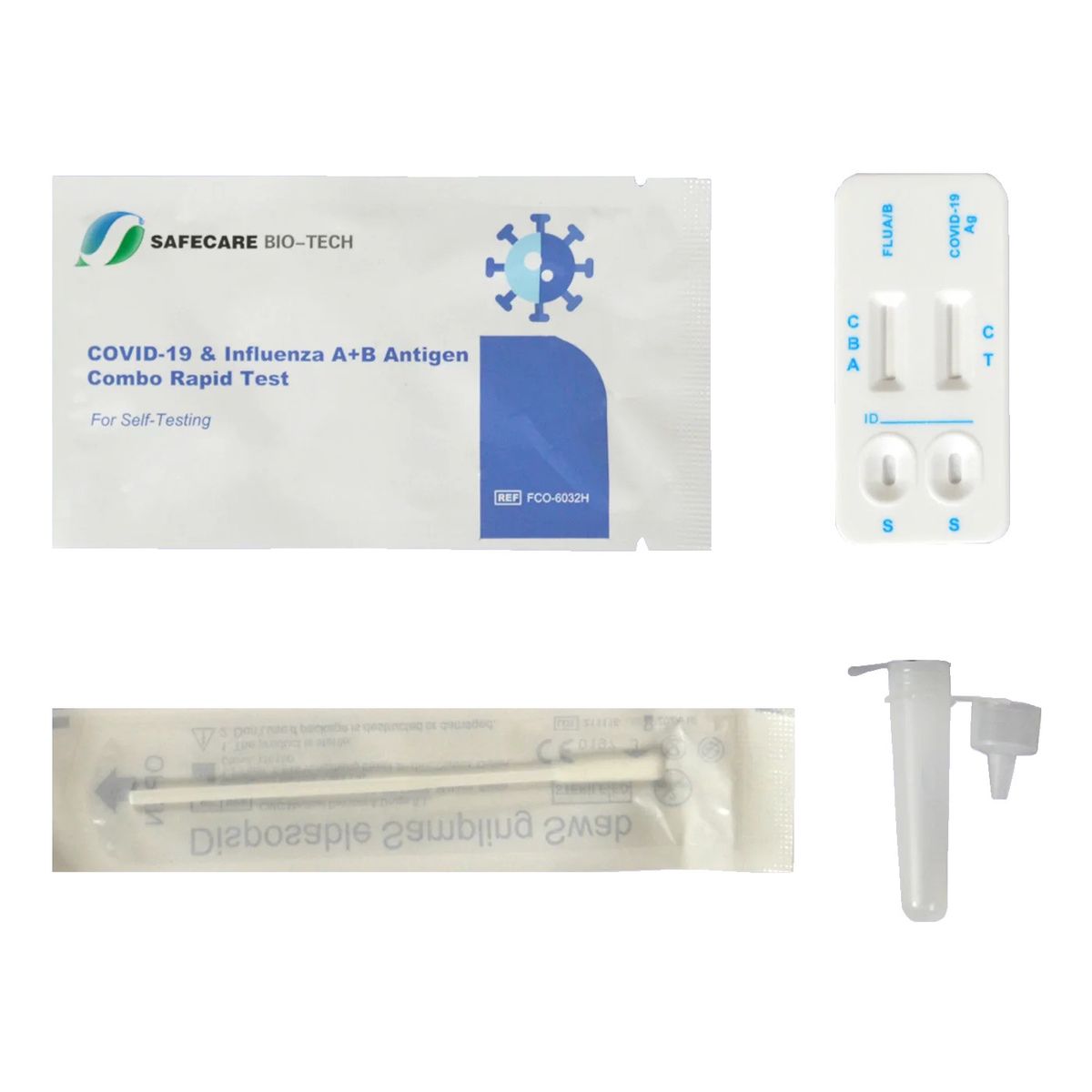
Safecare COVID-19 + Influenza A+B Antigen Combo Rapid Test
- Item number: 313
- Manufacturer: Safecare

Ready for immediate dispatch
You have questions?
Our trained contact persons will be happy to advise and help you.
The SAFECARE COVID-19 & Influenza Combo Antigen Test is an EU-certified test for public/home use.
This COVID-19 & Influenza A+B Antigen Combo Rapid Test is a disposable test kit for the qualitative detection of influenza A and B virus nucleocapsid protein antigen and COVID-19 antigen from nasal swab specimens.
This test is intended for home use with self-collected nasal swab specimens in persons 12 years of age and older. Sampling in persons under 12 years of age and over 70 years of age should be done under the guidance and assistance of a caregiver. Individuals who are unable to perform the test on their own should seek assistance.
The test is intended for symptomatic persons within 7 days of the onset of infection or for asymptomatic persons who have contact with persons who have been diagnosed positive or with persons suspected of being infected. Positive results indicate the presence of influenza and SARS-CoV-2. Individuals who test positive should self-isolate and seek additional treatment from their healthcare provider.
Product Features:
The SAFECARE COVID-19 & Influenza Combination Antigen Test is supplied in a convenient single pack.
This test detects all variants of COVID-19
Less invasive nasal swab - much more convenient than other tests
The SAFECARE COVID-19 & Flu Combination Antigen Test is clinically validated in the EU - unlike other tests, this is a rapid antigen test with clinical validation you can trust
The SAFECARE COVID-19 & Flu Combination Antigen Test comes with easy to follow instructions
Delivery time is 24/48 hours by courier.
For larger order quantities (>1,000), we can provide a higher discount - please contact shop@mundschutzhandel.de
After-sales support for the test:
E-mail: shop@mundschutzhandel.de
Phone: 05901-9585833
The SAFECARE COVID-19 & Influenza Virus Combo Antigen Test Kit is a one-step immunochromatographic in vitro assay. It is designed for the rapid qualitative determination of SARS-CoV-2 virus antigen in anterior nasal swabs from individuals with suspected COVID-19 within the first seven days of symptom onset. The COVID-19 Ag Test Kit should not be used as the sole basis for diagnosing or ruling out SARS-CoV-2 infection. Children under 14 years of age should be accompanied by an adult.
EASY TO USE: Simple sample collection by nasal swabbing.
FAST AND QUICK: Results in 15 minutes. Does not require instruments.
This product carries the CE-IVD mark for professional use and complies with the European Directive on In Vitro Diagnostic Medical Devices (98/79/EC) for home use.
Scope of delivery:
1x test cassette
1x Extraction tube with integrated buffer and tip
1x Sterile disposable swabs
1x Instructions for use
1x garbage bag
SUMMARY
The new coronaviruses belong to the β-genus. COVID-19 is an acute infectious disease of the respiratory tract. Humans are generally susceptible. Currently, patients infected with the new coronavirus are the main source of infection, but asymptomatic infected individuals may also be a source of infection. According to current epidemiologic studies, the incubation period is 1 to 14 days, usually 3 to 7 days. The main symptoms include fever, fatigue, and dry cough. Nasal congestion, runny nose, sore throat, myalgia, and diarrhea are observed in a few cases.
- Item number: 313
- Weight: 27g
- Content: 1 piece
- Dimensions: 122x67x22mm
- Manufacturer: Safecare
- Condition: New
Other customers also bought
 Add to wish list
Layman-Selftest
Detects Omicron
(2)
Acon
Acon FlowFlex Selftest €1.00
Add to wish list
Layman-Selftest
Detects Omicron
(2)
Acon
Acon FlowFlex Selftest €1.00 €1.25-20% €1.00€1.25-20% Delivery time on request Add to wish list Add to wish list
Layman-Selftest
Detects Omicron
(0)
Clungene
Clungene COVID-19 Antigen Rapid Corona Self Test - 5 pcs. €2.51
Add to wish list
Layman-Selftest
Detects Omicron
(0)
Clungene
Clungene COVID-19 Antigen Rapid Corona Self Test - 5 pcs. €2.51 €8.32-69% €2.51€8.32-69% €0.50 / piece €0.50 / piece Ready for immediate dispatch Add to wish list Add to wish list
Layman-Selftest
Detects Omicron
(0)
Lepu Medical
LEPU Medical NASOCHECK comfort self-test (pack of 1) €0.46
Add to wish list
Layman-Selftest
Detects Omicron
(0)
Lepu Medical
LEPU Medical NASOCHECK comfort self-test (pack of 1) €0.46 €0.83-44% €0.46€0.83-44% Delivery time on request Add to wish list
This might also interest you
Shipping costs
- Germany 5.80€
- Europe from 15,99€
- Switzerland from 26,99€
DHL Parcel
| bis 5kg | bis 10kg | bis 20kg | bis 31,5kg | |
| 15,99€ | 20,99€ | 31,18€ | 43,86€ | |
| 26,90€ | 34,99€ | 48,99€ | 55,99€ | |
| 29,99€ | 37,99€ | 52,99€ | 60,99€ |
DHL Express Parcel
| bis 5kg | bis 10kg | bis 20kg | bis 31,5kg | |
| 19,19€ | 25,19€ | 37,39€ | 52,59€ | |
| 34,90€ | 44,90€ | 59,90€ | 69,90€ | |
| 39,90€ | 49,90€ | 54,90€ | 74,90€ | |
| 39,90€ | 49,90€ | 69,90€ | 79,90€ | |
| 49,90€ | 69,90€ | 99,90€ | 129,90€ | |
| 49,90€ | 54,90€ | 89,90€ | 124,90€ | |
| 54,90€ | 74,90€ | 119,90€ | 159,90€ | |
| 64,90€ | 84,90€ | 129,90€ | 169,90€ |
all prices incl. VAT